ISO 13485 certification
We have a certified quality management system according to ISO 13485. Our products meet the requirements of Council Directive 93/42/EEC, ensuring a high level of testing and quality control. Production takes place in clean rooms classified as ISO 7 and 8.
MDR compliant
We have successfully completed an MDR audit and fully met the requirements of the MDR regulation, which enhances the safety and quality of medical products and devices.
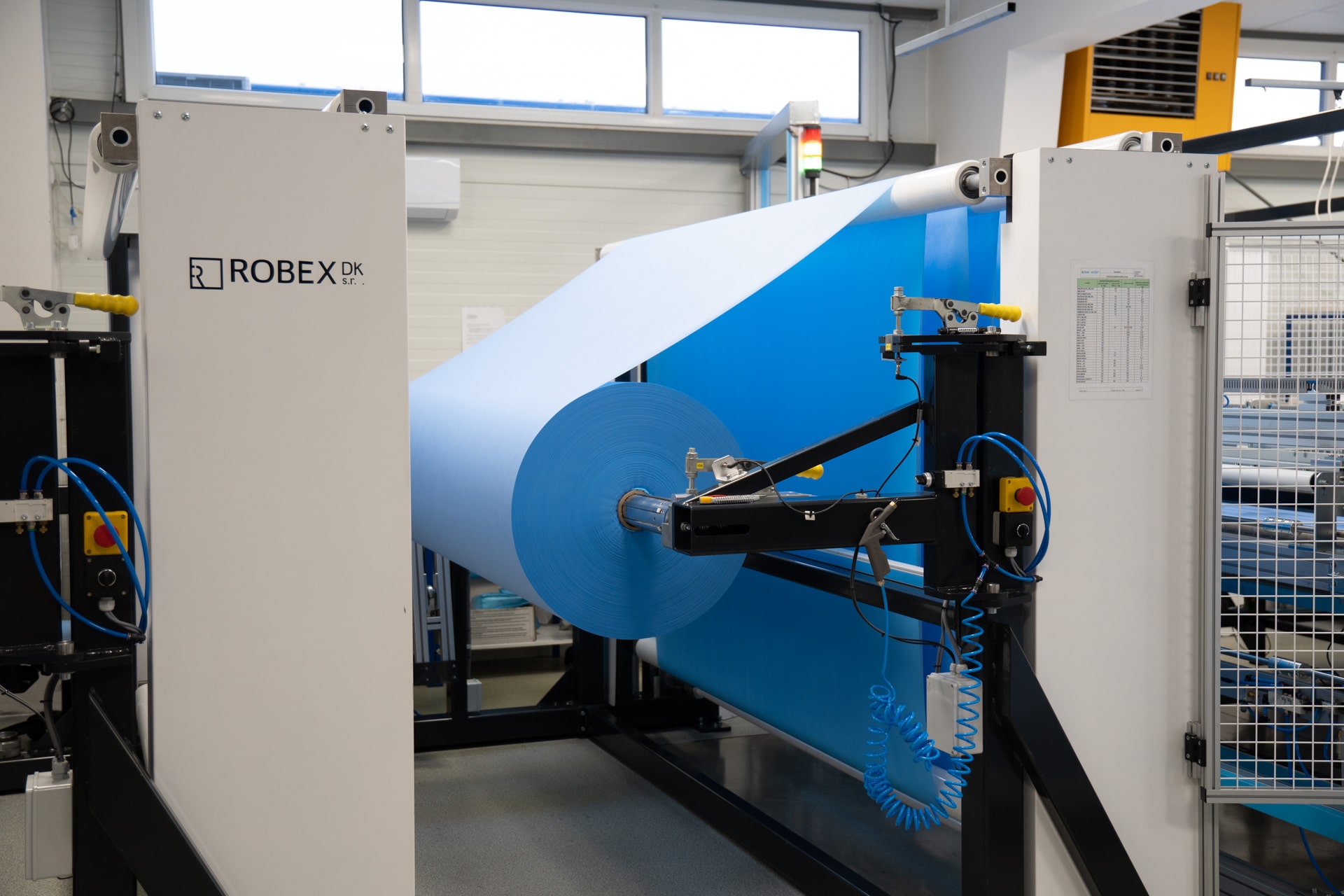
High-volume laminating line
Our laminating line processes 60,000 m² of two- and three-layer materials daily, achieving an optimal combination of properties for our products.
High-frequency fully automatic production line
We produce more than 12,000 drapes daily on a fully automated high-frequency line, ensuring efficiency and precision with every order. We also manufacture special drapes manually.
Testing laboratory
Continuous testing of the physical properties of materials and finished products takes place in our own laboratory to ensure the highest standards of quality.
Our ETO chamber
We have our own sterilization unit validated according to ISO 11135. Here, we sterilize surgical drapes, gowns, and smaller sets, ensuring the safety and sterility of our products.
Our modern laminating lines process both 2-layer and 3-layer laminated materials. After being cut into smaller rolls on our precise cutter, the materials are further prepared for subsequent processing steps and use. With an emphasis on efficiency and quality, we ensure fast and reliable production.
Interesting figures about our production:
• 6 types of laminates produced
• Daily production of 60,000 m²
• Annual production would wrap around the Earth 150 times
In our clean rooms certified to ISO 7, we manufacture various types of surgical drapes on a fully automated line. These drapes meet the strictest quality standards. For special needs, we produce highly complex drapes manually, which allows for customization of sizes, shapes, and accessories such as fixation elements or other specific modifications.
Interesting numbers about the automated line:
• More than 12,000 drapes daily
• Longest drape: 270cm
• Shortest drape: 50cm
We add additional components and products to the finished drapes according to precisely defined specifications and order. Our sterile surgical sets are designed for quick and efficient use directly in the operating rooms, ensuring maximum patient safety.
Surgical sets Dina-Hitex:
• Complete sterile sets ready for immediate use
• Customized assemblies according to individual customer needs
• High production flexibility ensuring quick delivery
Supplementary production programme
The core of the company is the manufacturing of surgical drapes and sterile surgical sets. However, we also have an extensive supplementary production program in an effort to offer as much variety as possible for the production of drapes and components for sets.
In our facilities, we produce:
Disposable garments for hospitals and operating rooms
Adhesive tapes
Pouches for fluid collection
Covers for medical devices, other plastic products
Packaging and sterilization technology
Our automatic packing line packages into both soft and hard blisters, thereby speeding up production. We have set validated cleaning processes for stainless steel tools according to the ISO 15883 standard to ensure maximum safety when using our tools.
We have our own sterilization unit validated in accordance with ISO 11135.
We conduct thorough testing of the physical properties of purchased materials and our own products in compliance with EU standards.
Our digitalized warehouse has a capacity exceeding 5000 Euro pallets.








